Documentation
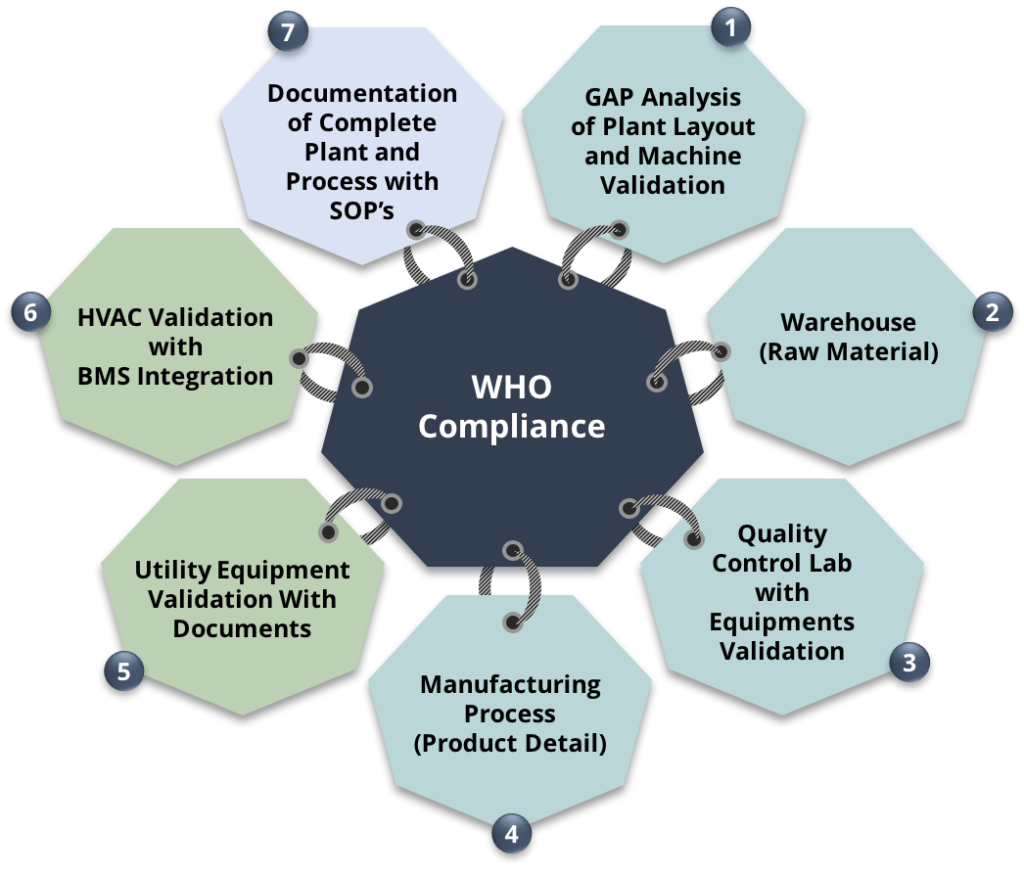 Regulatory Compliance for Upgradation of Existing Plant
Regulatory Compliance for Upgradation of Existing Plant
- Complete plant documentation work like SOP, Batch manufacturing records, Design Qualification, Installation Qualification, Operational Qualification, Performance Qualification.
- SOP for complete machinery, with utility system.
- SOP for cleaning procedure of filters, storage Tanks, HVAC System, Water System, Air Curtains etc.
Third Party / External Audits
- Technical / Engineering Audit of Pharmaceutical plant, which includes designing, utility criteria
- To check GMP, WHO compliance status of plant and its capability for future regulatory
- Review of Record, Documentation, Previous regulatory audit and Compliance report
- Prepare GAP analysis report
- Training for audit to the technical person



